
Authored by - Prashant Jawade, Saira Banu Pathan, Poonam Lalla
Published in - International Journal of Medical Microbiology and Tropical Diseases 2024 September, (3):292-295
DOI: https://doi.org/10.18231/j.ijmmtd.2024.050
ABSTRACT
Introduction: Malaria, caused by Plasmodium parasites, poses a significant global health challenge, demanding accurate and timely diagnostic approaches. This study investigates the identification of malaria parasites using peripheral smear and validate the same as the flaggings in Haematology analyzers specifically for Plasmodium Vivax is the most widespread of all of the malaria species, can cause severe, even fatal infections and results in significant global morbidity and mortality.
Materials and Methods: A cohort of 400 blood samples, evenly distributed between male and female participants, underwent analysis for the presence of the Trophozoite Form of Plasmodium vivax on peripheral smears and then on Erba 5 part Haematology analyzers Elite 580 & H560 to detect the flagging.
Results: The statistical analysis of trophozoite-positive malaria samples on both analyzers demonstrated a significant association between the two analyzers and the manual peripheral smear method. The specificity analysis revealed high accuracy for both haematology analyzers - H560 (92.95%) and Elite 580 (88.14%) in correctly identifying trophozoite-negative samples.
Conclusion: This study contributes essential insights into malaria diagnosis, emphasizing the need for flaggings in the CBC results. The observed specificity values and concordance between haematology analyzers provide valuable information for optimizing malaria diagnostic strategies in diverse settings.
1. INTRODUCTION
Malaria, a life-threatening infectious disease caused by Plasmodium species, remains a significant global health challenge, particularly in endemic regions such as the continents of Africa and Asia as per the information released by Centres for Disease Control and Prevention.1 Human malaria is caused by one or more of four parasites: Plasmodium falciparum, P. vivax, P. ovale, and P. malariae. Distribution of these parasites varies geographically, and not all species of malaria are transmitted in all malarious areas. P.vivax malaria, however, is the predominant species in Central America, most of malarious South America, and the Indian subcontinent (Miller et al., 1977).
Accurate and timely diagnosis is pivotal for effective disease management, treatment, and control strategies. Various diagnostic methods, including microscopy, rapid diagnostic tests (RDTs), and polymerase chain reaction (PCR), play crucial roles in identifying malaria parasites and guiding appropriate interventions. The choice of diagnostic tools is influenced by factors such as accessibility, cost, and sensitivity.
In recent years, a plethora of research has focused on optimizing and evaluating different diagnostic techniques to enhance their sensitivity, specificity, and overall performance. The utilization of RDTs, for instance, has gained prominence due to their simplicity, rapidity, and applicability in resource-limited settings.2,3 However, challenges such as the prozone effect have been identified, demanding a deeper understanding of their limitations and potential false-negative results.4 Advancements in molecular techniques, particularly PCR-based methods, have offered higher sensitivity and specificity in malaria diagnosis.5 These techniques have proven valuable in confirming infections, especially in cases where RDTs
may yield false-negative results, thus highlighting the complementary roles of different diagnostic approaches. Moreover, the epidemiology of malaria varies across regions, influencing the performance and applicability of diagnostic tools.6,7 In India, where malaria remains endemic, understanding local transmission dynamics and the prevalence of asymptomatic carriers is essential for refining diagnostic strategies.8
These two Analyzers have been chosen for their relevance and prevalence in malaria-endemic areas, offering insights into their performance characteristics.6 Through a comprehensive analysis of these samples, we aim to contribute valuable information to the on-going discourse
on malaria diagnosis, with a particular focus on the specificity and accuracy of Elite 580 & H560 in the context of a diverse sample set. 6,8
2. MATERIALS AND METHODS
2.1. Study design
A cohort study consisting of 400 blood samples collected from 400 patients - 200 male and 200 female who visited the Medicine OPD at Navi Mumbai Municipal Hospital (NMMC), Mumbai, Maharashtra, India, with probable symptoms of Malaria such as Fever with chills past 1-2 days, vomiting, fatigue and headache were included in this study. The objective was to analyse the presence of the trophozoite form of P.vivax with peripheral smear and access the specificity of two haematology analyzers Elite 580 & H560.
2.2. Sample collection
Blood samples were collected as per the Ethics with demographic details of each participant during the tenure June 2023 to Sept 2023.Consent for participation in the study was taken from the Laboratory authority. Since the portion of blood samples utilized for the study was taken from the leftover blood samples and not withdrawn separately for this study, participants consent was not required. However the confidentiality of patient history & details was strictly maintained. Inclusion criteria was simple, samples that were positive for Malaria on peripheral smear were included. Similarly peripheral smears negative results sample were not included in the study.
The gold standard in the diagnosis of malaria is detection of parasites by microscopic observation of Giemsa-stained blood smears.9,10 The ratio of Plasmodium infected red blood cells (RBCs) can be calculated, and indeed, Plasmodium species can be identified according to their morphological characteristics. However, the sensitivity and specificity of the diagnosis depend on the skill of the microscopist.
Diagnosing malaria clinically relies on symptoms like fever and chills, which are nonspecific, making accurate diagnosis challenging. Currently, microscopic detection of the malaria parasite remains the gold standard despite its cost-effectiveness, yet it suffers from subjective interpretation of smears. Expertise is crucial for accurately interpreting peripheral smears (PS), particularly in cases with low parasitic indices, where reliability diminishes. Therefore, laboratory errors may occur. Hematology analyzers are increasingly employed in labs, automating the analysis of various parameters. The normal scattergram (Figure 1) on the differential (DIFF) plot includes five components: lymphocytes (pink), monocytes (green),
neutrophils and basophils (blue), eosinophils (red), and a distinct space separating the neutrophil and eosinophil populations.
Modern automated analyzers can now detect malaria parasites through careful observation of scattergram. It has been observed that hemazoin pigment produced by malaria parasites can scatter laser light, leading to abnormal scattergram patterns in complete blood count (CBC) analysis. Various abnormal patterns seen on WBC-DIFF (differential) scattergram (Figure 1) in malaria infection include mixing of neutrophil and eosinophil clusters, double neutrophil or eosinophil clusters, greying of neutrophil and eosinophil clusters, reduced spacing between eosinophil and neutrophil clusters, large eosinophil clusters, and prominent blue/purple events above the X axis.11
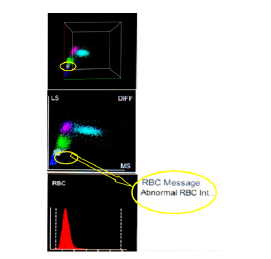
Figure 1: Scattergram showing the area with Infected RBCs
Hemozoin pigments produced by the malaria parasite have the ability to alter the polarization of laser light. As a result, infected red blood cells displaying various morphological forms such as trophozoites (ring forms), schizonts, and gametocytes, as well as phagocytic cells (monocytes, macrophages, and neutrophils containing the parasite), generate abnormal and unusual scattergrams during routine CBC analysis. The current study aimed to evaluate the effectiveness of WBC scattergrams produced by the Erba Fully Automated Hematology analyzers Elite580 & H560 in diagnosing malaria, with the goal of enabling early detection and treatment to prevent subsequent complications.11 Although these automated
analyzers have significant potential to screen the malaria parasite, these analyzers are not widely available in Basic Primary healthcare set ups in remotely located laboratories in India and this limitation hinders its integration and usage into routine care practice.
Thin and thick blood smears were prepared from each blood sample and stained with Giemsa stain.12 Experienced microscopist examined the stained slides under a light microscope to identify and quantify the trophozoite form of P.vivax. The positive samples of P.vivax were checked on both Erba analyzers - Elite580 & H560 to capture the flaggings. The presence or absence of trophozoites in each sample was recorded for both the analyzers. The collected data was analysed to determine the specificity which was calculated based on four categories: trophozoite-positive in both Elite 580 & H560, trophozoite-positive in H560 and negative in Elite 580, trophozoite-negative in H560 and positive in Elite 580, and trophozoite- egative in both Elite 580 & H560.Descriptive statistics, such as frequencies, percentages, chi-squared value and odds ratio, were used to summarize the findings. The specificity of each analyzer was calculated as a percentage. The SPSS version 15.0 used for the statistical analysis.
3. RESULTS
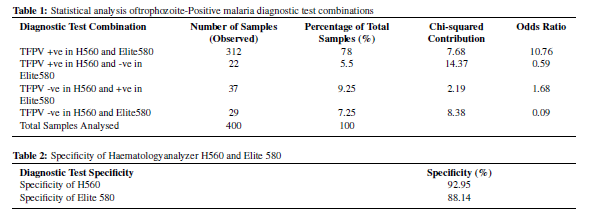
In this study, among the trophozoite-positive samples, Out of total 400 blood samples which were all positive on peripheral smear, 312 samples tested positive for trophozoites in both analyzers. While for 22 samples, H560 identified trophozoites while Elite580 did not. For 37 samples, Elite580 identified trophozoites while H560 did not. And for 29 samples, both H560 and Elite580 could not identify the trophozoites which were present in the smears.
TFPV +ve in H560 and Elite580 both (312 samples, 78.00%): A significantly higher likelihood (odds ratio = 10.76) of concordant positive results in both H560 and Elite580 was observed (Table 1).
TFPV +ve in H560 and -ve in Elite580 (22 samples, 5.50%): A decreased likelihood (odds ratio = 0.59) of discordant results, suggesting that if H560 identifies trophozoites, Elite580 is less likely to miss them. (Table 1)
TFPV -ve in H560 and +ve in Elite580 (37 samples, 9.25%): A moderate likelihood (odds ratio = 1.68) of discordant positive results between H560 and Elite580 was observed. (Table 1)
TFPV -ve in H560 and Elite580 both (29 samples, 7.25%): A significantly reduced likelihood (odds ratio = 0.09) of discordant negative results between H560 and Elite580 was noted. (Table 1)
The specificity of the H560 analyzer was determined to be 92.95%, signifying its high accuracy in correctly identifying trophozoite-negative samples. On the other hand, the specificity of the Elite 580 analyzer was found to be 88.14%, indicating its effectiveness in correctly identifying trophozoite-negative samples. (Table 2)
These results provide valuable insights into the diagnostic performance of H560 and Elite580 in identifying trophozoite-positive and trophozoite-negative samples within the studied cohort. The findings contribute to our understanding of the efficacy of these analyzers in the context of Plasmodium vivax detection.
4. DISCUSSION
The findings of the present study, which focused on the identification & flagging for Plasmodium vivax resonate with existing research on malaria diagnostics. The prevalence of P.vivax observed in all analysed samples aligns with the well-established fact that the trophozoite stage is a crucial target for malaria diagnosis due to its presence during the symptomatic phase of the infection.2
The high concordance of trophozoite-positive results on Elite580 & H560 as flaggings on 312 samples emphasizes their effectiveness in detecting P.vivax infections, corroborating the findings of other studies that have evaluated the performance of rapid diagnostic tests (RDTs) in malaria-endemic regions.6 However, the discrepancies observed in the results, might be attributed to variations in test sensitivities, the presence of low parasitaemia, or specific characteristics of the study population.3,8,13 The variations in specificity might be influenced by factors such as geographic location, the prevalence of different Plasmodium species, and the potential impact of genetic variations in the parasite population.7,14
Moreover, the importance of understanding the local epidemiology of malaria, as emphasized by Singh et al.,7 is underscored by the results of this study. The prevalence and distribution of Plasmodium species, as well as the performance of diagnostic methods, can vary significantly between regions, influencing the choice of appropriate diagnostic tools for effective malaria control strategies.5,15
5. CONCLUSION
In conclusion, our study revealed the presence of P. vivax as flagging on Elite580 and H560 with specificity values of H560 (92.95%) and Elite580 (88.14%) aligning with existing research, emphasizing the need for context-specific interpretation. This study contributes essential insights for refining malaria diagnostic strategies, crucial for tailored and effective control measures.
6. ETHICAL APPROVAL
The study was approved by the Ethical Committee of Institute, Ethical approval number EC-NMMC-2023/01/117
7. CONFLICT OF INTEREST
No Conflict of interest was observed.
8. SOURCE OF FUNDING
None.
9. ACKNOWLEDGEMENT
The authors wish to thank Archana Ravindranath & Deepak Valand, from Transasia Biomedicals Ltd for providing the technical support in data capture and analysis.
REFERENCES
- Malaria’s Impact Worldwide. Available from: https://www.cdc.gov/malaria/php/impact/?CDC_AAref_Val=https: //www.cdc.gov/malaria/malaria_worldwide/impact.html.
- Maltha J, Gillet P, Jacobs J. Malaria rapid diagnostic tests in endemic settings. Clin Microbiol Infect. 2013;19(5):399–407.
- Valecha N, Bhatia S, Mehta S. Epidemiology of Plasmodium vivax malaria in India. Am J Trop Med Hyg. 2009;81(5):837–9.
- Anvikar AR, Arora U, Sonal GS, Mishra N, Shahi B, Savargaonkar D, et al. Antimalarial drug policy in India: past, present & future. Indian J Med Res. 2014;139(2):205–15.
- Moonasar D, Morris N, Kleinschmidt I. What will move malaria control to elimination in South Africa? S Afr Med J. 2013;103(10):801–6.
- Sharma SK, Chattopadhyay R, Chakrabarti K, Pati SS, Srivastava VK, Tyagi PK, et al. Epidemiology of malaria transmission and development of natural immunity in a malaria-endemic village. Am J Trop Med Hyg. 2004;71(4):457–65.
- Singh V, Mishra N, Awasthi G, Dash AP, Das A. Why is it important to study malaria epidemiology in India? Trends Parasitol. 2009;25(10):452–7.
- Sonal GS, Shweta P, Swaraj B. Insights into malaria diagnosis under high transmission settings: Implications for elimination. Indian J Med Microbiol. 2017;35(3):323–9.
- Nguyen PH, Day N, Pram TD, Ferguson DJ, White NJ. Intraleucocytic malaria pigment and prognosis in severe malaria. Trans R Soc Trop Med Hyg. 1995;89(2):200–4.
- Maru AM, Shrivastava A, Chokshi T, Agnihotri AS. Utility of automated hematology analyzer in diagnosis of malarial parasite. Indian J Pathol Onco. 2019;6(3):428–33.
- Bute RS, Bharambe BM, Pawar R, Jadhav AB. Abnormal WBC Scattergrams by Sysmex XN550, A Supplementary Diagnostic Tool for Malaria to the Conventional Methods. Ann Pathol Lab Med. 2021;8(1):14–9.
- Moody A. Rapid Diagnostic Tests for Malaria Parasites. Clin Microbiol Rev. 2002;15(1):68–78.
- Koita OA, Doumbo OK, Ouattara A, Tall LK, Konaré A, Diakité M, et al. False-negative rapid diagnostic tests for malaria and deletion of the histidine-rich repeat region of the hrp2 gene. Am J Trop Med Hyg. 2012;86(2):194–8.
- Johnston SP, Pieniazek NJ, Xayavong MV, Slemenda SB, Wilkins PP, Silva AD, et al. PCR as a confirmatory technique for laboratory diagnosis of malaria. J Clin Microbiol. 2006;44(3):1087–9.
- Vásquez AM, Medina AC, Tobón-Castaño A. Evaluation of the diagnostic accuracy of CareStart G6PD deficiency Rapid Diagnostic Test (RDT) in a malaria endemic area in Colombia. PLoS Negl Trop Dis. 2016;10(4):4457. doi:10.1371/journal.pntd.0004457.
Author Biography
Prashant Jawade, Medical Superintendent
Saira Banu Pathan, HOD
Poonam Lalla, Family Physician Ayurveda https://orcid.org/0000-0003-1666-3135