Latest Advancements in Coagulation Analyzers
Introduction
Huang Ti, the Chinese emperor, first described the length of bleeding time around 3000 years ago. It was in the year 1628 when William Harvey scientifically proved blood circulation and in 1905 the phenomenon of coagulation was first put forward by the German scientist Paul Morawitz. In 1960, the coagulation cascade was described which led to the development of the first clotting time tests1.
Genesis of automation
Coagulation assays principally include the Prothrombin Time (PT) test, which measures the tissue factor-induced clotting time of plasma, the activated Partial Thromboplastin Time (aPTT) (commonly used as screening tests for monitoring unfractionated heparin (UFH) therapy), and the Thrombin Time test (TT), an assay measuring the conversion rate of fibrinogen to insoluble fibrin after the addition of thrombin to plasma(1).
The semi-automated coagulometer was introduced in the 80’s. The earlier assay methods were based on the manual visual detection of a clot, either through observation of fibrin strands or the detection of bulk changes in viscosity. Gradually, the visual detection of clot formation method was replaced by optical detection technologies; turbidimetric and nephelometric technology. The visual detection method was later replaced by mechanical detection in which the change in viscosity was detected with help of the movement of a steel ball in the rotating sample tube(1).
The automation of coagulation methods led to many benefits like high volume testing, better reproducibility, user flexibility, time and cost reduction, and better precision with quality controls. Coagulation instruments gradually evolved by adding different technologies to the same instrument. Those technologies were optical detection of clot formation, chromogenic substrates, latex agglutination immunoassays and turbidimetry.
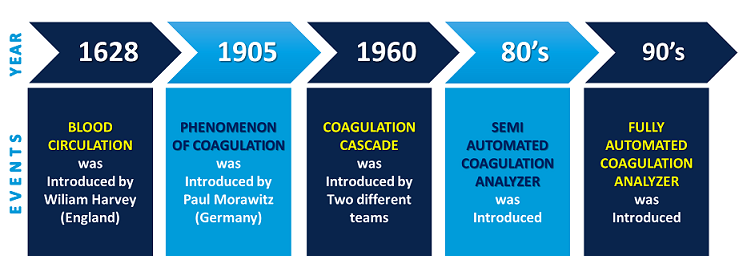 Figure 1: Timeline for the evolution of the coagulation analyzer
Figure 1: Timeline for the evolution of the coagulation analyzer
Alternate technologies
The recently introduced technologies are: Luminescent Oxygen Channelling Immunoassay (LOCI), magnetic sensor based Point of Care (POC) coagulation testing, microfluidic strip based POC, microresonators based on Microelectromechanical systems (MEMS) technologies and electrochemical sensor based coagulation testing. Along with these In-vitro techniques, Campbell et al. introduced Laser Scanning Confocal Microscopy (LSCM) to observe in situ thrombin generation. Other In-vivo studies of thrombin introduced microenzyme immunoassay to measure thrombinantithrombin complex (TATc) levels.
Since the beginning of the new millennium, the coagulation testing has evolved rapidly due to faster technology development and increase in demand of quality testing by users. Advanced research has seen an inclination towards coagulation tests with POC, primarily focussed on improved systems miniaturisation and functional integration. But POC technologies are facing challenges which include the quality, robustness, cost, intellectual property issues, regulatory approval, clinical acceptance, the economics of test usage and the ultimate market size(1).
Future trends
In April 2019, Favaloro et al published a review on mainstream hemostasis analyzer2. The study highlighted future innovations that would be desirable by the user, they are:
- Fully Automation
- Integration with pre- and post-analytical platforms and full integration with Laboratory Information Systems (LIS integration)
- Modular analytics (“stackable instruments” or several modules side by side enabling PROLONGED throughputs and continued throughput in case of issues with one module)
- Ready to use, long-term stability reagents
- Expansion of interference detecting systems
- Different assay technologies on single instruments
- Expanded capacity for automatic re-run, reflex testing
- PROLONGED quality with affordability
At Transasia Bio-Medicals Ltd, technology advancement and constant improvement are the focused points. We have matched and superseded the current user requirements with our Advanced Fully Automated Random Access Coagulation Analyzer ECL 760 which is inbuilt with different technologies in one instrument.

Features of ECL 760, with current user requirement are as follows:
- Fully automated, Random access (60 PT Tests / Hour)
- On board reagent refrigeration & storage
- Advance, simple and robust reagent chemistry
- Smaller foot print (66cms x 58cms x 51cms)
- Covers all coagulation parameters including “D-Dimer”
- Different methods of end point detection on instrument with LED based detection optics
- Scatter Light Detection
- Colorimetry
- Latex Enhanced Turbidimetry
- Minimal sample volume (Ranges from 5 µL to 50 µL)
- Clot detection curve monitoring
- Highly precise with L-J chart availability (12 Levels)
- Manual and automated calibration
- 6 point calibration
- Internal Quality Control (IQC) available (4 types)
- External Quality Assessment System (EQAS) compatible
- Re-run and reflex testing facility available
- 27 sample at a time with STAT sample run facility
- Ease of reagent management
- 8” LCD operating touch screen
- Window based system can be operated by touch screen, external keyboard and mouse
- Unlimited data storing capacity
- Data transfer with USB possible
- LIS bidirectional interfacing possible
- Affordable consumable
- Reagents with long open vial stability
References:
- Leanne F. Harris; Coagulation Monitoring Devices: Past, Present, and Future at the Point of Care; Trends in Analytical Chemistry, Vol. 50, October 2013.
- Favaloro EJ, Lippi G; Recent Advances in Mainstream Hemostasis Diagnostics and Coagulation Testing; Semin Thromb Hemost. 2019 Apr;45(3):228-246.