The Significance of Quality Control in Coagulation
Quality Council of India (QCI) is an autonomous body of the Department of Industrial Policy and Promotion, Government of India. The main objectives of QCI are to establish and operate a national accreditation structure and to monitor and administer the National Quality Campaign. National Accreditation Board for Testing and Calibration Laboratories (NABL) which currently are under the purview of the Department of Science and Technology (DST) has a unique role to play in the QC operations. As per the latest updated list of NABL, more than 1000 laboratories have acquired NABL certification for ISO 15189:2012 standards. This number is slowly increasing which indicates that more and more awareness is being created in smaller to larger pathology laboratories about patient safety and precise laboratory operations.1-2
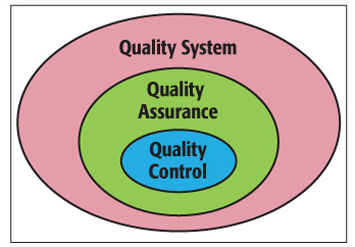
Clinical laboratories are using various methods to reduce errors, ensure patient safety, and improve quality. Due to advances in analytical techniques, automation in laboratory instrumentation, and information technologies, there is a significant decrease in analytical error rates. Laboratories thus can focus more on pre-analytical and post-analytical processes to improve patient safety. Yet analytical quality is a major challenge for clinical laboratories in the routine laboratory operations. The two main aspects of quality management are Quality Assurance (QA) and Quality Control (QC). The term, ‘Quality Control’ (QC) refers to the statistical quality control that is used in laboratories to monitor the routine performance of testing processes, to detect possible errors, and to rectify any problems before the test results are reported.3
Laboratory testing is carried out through various segments, for the purpose of diagnosis and treatment of patients with various clinical disorders and diseases. Coagulation is another segment of clinical diagnosis that can be explored for quality control for the various coagulation parameters. The available research or published data is mainly represented by the QC related to Prothrombin Time (PT) Point-of-care Testing (POCT) and some are focused on the importance of pre-analytical variables. The available published data clearly highlights that patient identification, specimen collection and handling, often represent neglected procedures in health care, involving serious risk of errors. It is very important in coagulation testing to standardize the analytical procedure and monitor the preanalytical variables for reliable results. Hence, it is necessary to include an effective quality control and quality assurance program in all procedures and processes involved.
According to published data, this model of imprecision (random error), bias (systematic error) and total error plays a key role in setting performance characteristics. Westgard and Westgard have assessed the quality of laboratory testing in the United States, using the Six Sigma metrics. As per this metric, the higher the σ metric, the better the quality. The quality products should have a quality performance value of 6 s, and minimum acceptable process capability should correspond with to a 3S process. The authors calculated three estimates of quality from the data of proficiency testing programs. The first is National Test Quality (NTQ) for random and systematic error, the second is National Method Quality (NMQ) for bias and Coefficient of Variation (CV) and the third is the Local Method Quality (LMQ) for CV determination without accounting for method bias; all of them are estimated from the data available with the Clinical Laboratory Improvement Amendments (CLIA). This data calls for improvement in quality control programs, including an increased number of controls per run and the adoption of control rules selected to maximize error detection.3
It is necessary for every lab to perform daily run Internal Quality Control (IQC) to achieve desirable quality results. The External Quality Assurance System (EQAS) or Programs (EQAP) help enhance patient care and safety, through improved laboratory practice. EQAS are accreditation and regulatory requirements for NABL or ISO 15189 standardization. Due to vast changes in the pattern of tests employed and the technologies developed in clinical laboratories in the field of coagulation, various EQAS programs are facing challenges.
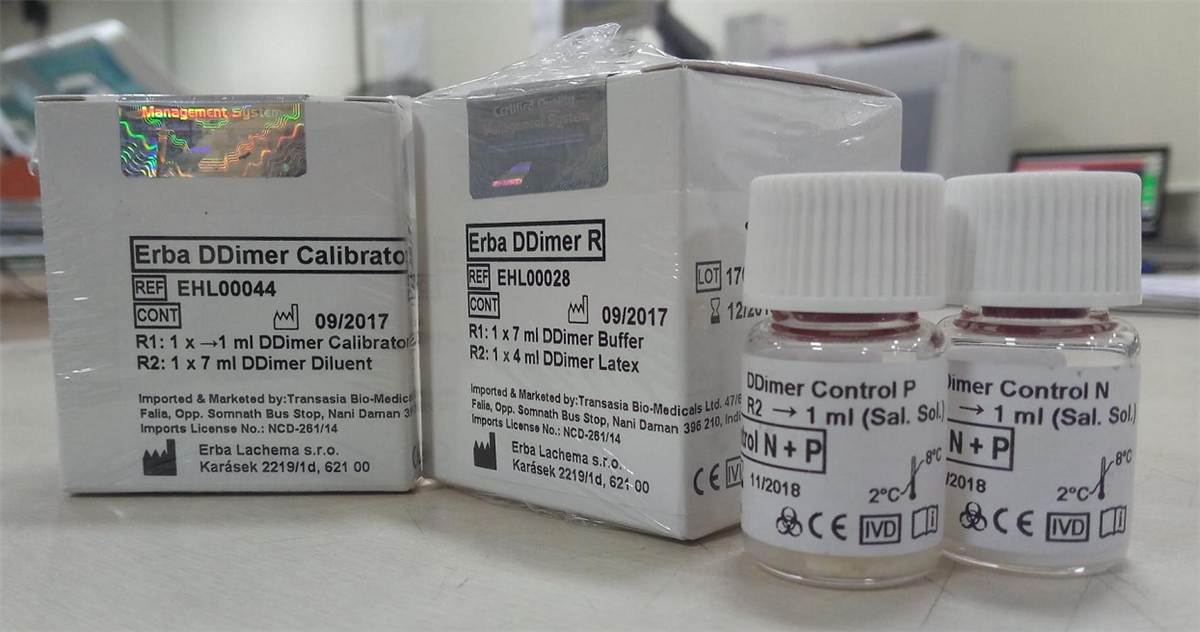
Transasia’s Erba Coagulation Line (ECL) quality control reagents can support laboratories to achieve excellent IQC results. We have four quality control reagents namely; Control N (Cat no. EHL00014), Control P (Cat no. EHL00015), Control N Plus (Cat no. EHL00016) and Control P Plus (Cat no. EHL00017). Control N and Control P can be used for IQC of Prothrombin Time (PT), Activated Partial Thromboplastin Time (APTT), Fibrinogen, Thrombin Time (TT) and Antithrombin III (AT III). Whereas, Control N plus and Control P plus includes various coagulation factors along with PT, APTT, TT, Fibrinogen and AT III. The lot wise data is maintained and provided with every lot of reagents for IQC reference. Our D Dimer has Erba DDimer Control N + P (Cat no. EHL00019) with high and low-level controls.
References:
- www.qcin.org
- www.nabl-india.org
- Mario Plebani, et al; Quality Control in Coagulation Testing; SEMINARS IN THROMBOSIS AND HEMOSTASIS/VOLUME 34, NUMBER 7 2008.