Assessment of Fibrinogen and Factor VIII levels in Fresh Frozen Plasma: A study
Introduction:
Uncontrolled hemorrhage of any etiology is a lethal disorder. This may lead to coma, seizure, organ failure, and ultimately loss of life. Appropriate hemorrhagic shock management by skilled and experienced healthcare professionals can prevent these lethal episodes. The pathophysiology of hemorrhage is based on the development of trauma-induced coagulopathy1. One of the effective treatments for hemorrhage is the transfusion of Fresh Frozen Plasma (FFP). Transfusion of FFP helps in replacing various coagulation factors that are lost during hemorrhagic shock as well as diseases like liver diseases or disseminated intravascular coagulation(2).
The high demand for FFP is challenging for routine blood bank activities. This high demand requires the inclusion of quality management activity during FFP management in the blood bank. Providing the right blood to the right person at the right time and place is the main aim of any quality management activity of a blood bank(2). The National AIDS Control Organisation (Ministry of Health and Family Welfare) has set standards to maintain quality management for routine blood bank activities. As per the guidelines, 1% of the total number of units prepared, or 4 units per month are tested for the quality assurance of FFP. This activity tests the stable coagulation factors that include fibrinogen (200 – 400 mg/dL) and factor VIII (55 – 145 %)3. The fibrinogen and factor VIII levels in FFP can be measured with the help of clotting assay on coagulation analysers (semi or fully-automated). The regular analysis and study of the quality of FFP is an essential activity for any blood bank(2).
Material and methods:
The data was collected from Smt. S. R. Mehta & Kikabhai Blood Bank, Sion from 1st January 2019 to 31st August 2019. During this timeframe, 1394 samples were processed. These samples are collected in sterile double/ triple bags from healthy individuals with written and signed informed consent from them.
Preparation of FFP:
The collected blood samples were centrifuged at 2200 rpm for 10 minutes after blood grouping. The primary bag containing centrifuged blood was placed on a plasma extractor and attached to the satellite bag on a dietary scale adjusted to zero. The plasma was expressed into the satellite bag and weighed before further processing. The transfer tubing was sealed with a dielectric sealer or metal clips without disturbing the segment number of tubing. Another seal was placed near the transfer bag and labeled the ‘satellite bag’ with the unit number prior to separation from the mother bag and the volume of plasma was recorded on the label. The segments were then available for reverse grouping and other tests. The processed plasma was stored at -80°C within 6 hours of collection of the donor unit. The packed cells were stored in the blood bank fridge at 4°C.
Estimation of Fibrinogen (FBG) and Factor VIII (F VIII):
The archived FFP was processed for estimation of FBG and F VIII on ECL 760 - Fully Automated Random Access Coagulation Analyser (Figure 1) from Transasia Bio-Medicals Ltd., India. The estimation of FBG was done by Clauss Method4 whereas for F VIII the One-stage method was used by measuring deficiency of the factor in substrate plasma with the help of estimation of Activated Partial Thromboplastin Time (aPTT)5. For FBG assay lyophilised Erba Thrombin Reagent was used which was reconstituted in 1mL distilled water before use. For F VIII lyophilised Erba Factor VIII deficient plasma was used. In F VIII assay, Erba Actime and Erba Calcium Chloride were used for aPTT estimation. Both analyses were done on ECL 760 Fully Automated Random Access Coagulation Analyser. On ECL 760 reagents were loaded and pre-programmed assays were performed by giving the command via touch screen. Both the assays were standardised with the help of Erba Standard Plasma and routine quality control was done by using the specialised Erba Control N Plus (normal control) and Erba Control P Plus (abnormal control). The dilution of controls, standard, and plasma samples was done by Erba Owren’s Vernol Buffer. The samples were processed only when the control values were found in range, failure of which led to the standardisation of the assay once again.
 Figure 1: ECL 760 - Fully Automated Random Access Coagulation Analyser
Figure 1: ECL 760 - Fully Automated Random Access Coagulation Analyser
Statistical analysis:
The statistical analysis was done by the Product Management Team of Transasia Bio-Medicals Ltd, Mumbai using Microsoft excel 2010.
Results:
Total 1394 samples were processed during the stipulated time period. Among these samples 357 viable plasma samples were issued for the treatment of hemorrhagic disorders and 68 plasma samples were kept in storage. The statistical analysis was done for these 419 samples. All the remaining samples were rejected from the study due to low levels of FBG or F VIII or due to unavailability of data. Samples having FBG value > 400 mg/dL was kept in the archive for further analysis. The samples were further differentiated using blood as mentioned in Figure 2. It was clearly observed that maximum samples were of blood group B Positive (138 samples) and samples for AB Negative blood group was very less in number (3 samples).
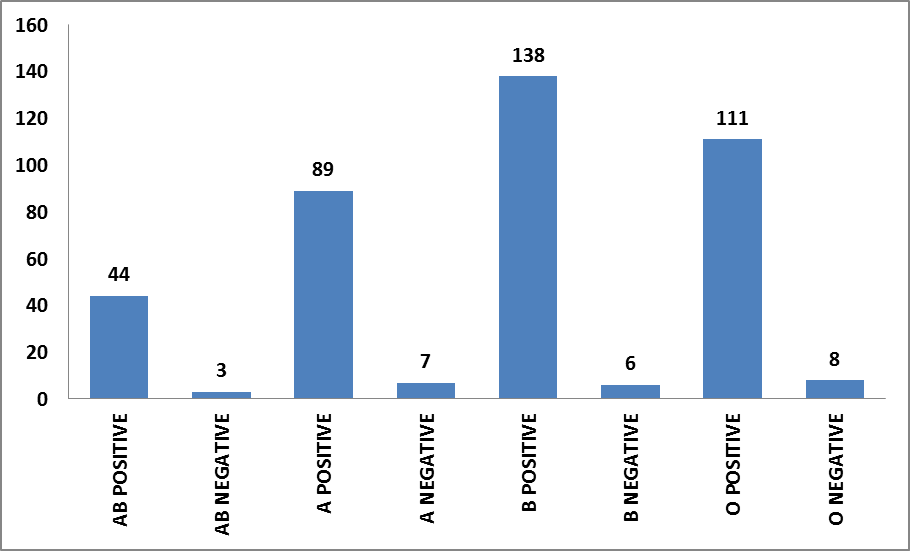 Figure 2: Differentiation of samples on the basis of blood groups
Figure 2: Differentiation of samples on the basis of blood groups
The fibrinogen value average is 283.28 mg/dL with a range of 200.3 to 471.1 mg/dL. The F VIII value average is 86.39 with range of 50.3 to 171.01 %. (Table 1)
Table 1: Range of values for FBG and F VIII
| |
Average |
Low |
High |
| Fibrinogen (mg/dL) |
283.28 |
200.3 |
471.1 |
| Factor VIII (%) |
86.39 |
50.3 |
171.01 |
The fibrinogen values were further differentiated as mentioned in Figure 3, where maximum number of samples had values between 251 – 300 mg/dL. The factor VIII values were differentiated as mentioned in Figure 4 where maximum number of samples had values between 76 – 100%.
Figure 3: FBG value differentiation
Figure 4: F VIII value differentiation
Discussion:
Therapeutically, transfusion of the FFP procedure is commonly used during severe bleeding episodes, or prophylactically in invasive procedures like surgery for non-bleeding patients. These coagulopathies may include liver diseases, vitamin K related coagulopathies, dilutional coagulopathy or Disseminated Intravascular Coagulation (DIC). Acute phase protein like F VIII has a tendency to rebound rapidly, during coagulopathies. The efficient quality management of blood component including FFP is effective in blood bank routine activity management and eventually betterment of patients(6).
Our aim at Smt. S. R. Mehta & Kikabhai Blood Bank, Sion was to maintain the high quality standards for FFP inventory management. All national and international guidelines have been considered for every blood bank procedure. In our study we assessed the thawed frozen plasma for fibrinogen and Factor VIII analysis. While going through references, we came across three similar kinds of studies. The first study was from Sultan et al, in which 100 samples were assessed for FBG and F VIII. In this study all patients had FBG values >150 mg/dL and desired F VIII values except 5% samples. In the second study which was documented by Agus et al, 30 units of FFP were prepared within 8 hours of collection and were assessed for FBG and F VIII, Dogra et al analysed 100 units of FFP for FBG and F VIII. In this study levels of fibrinogen were 270.66 ± 69.64 mg/dl and factor VIII were 117.205±29.01%2.
All these quality control assays where performed on Transasia’s ECL 760 – the Fully Automated Random Access Coagulation Analyzer. This instrument played a key role in maintaining high accuracy and precision during the study program. Wide range of reagents, excellent repeatability and ease of operation were the essential factors for our study. We recommend similar kind of studies to be performed on semi-automated instruments for the reference of blood banks with lesser number of samples. Even the temperature variation at the blood bank storage facilities should be analysed with well-designed studies.
Conclusion:
In conclusion we at Smt. S. R. Mehta & Kikabhai Blood Bank, Sion maintained national and international standards while preparation, archiving and analysis of FFP. Regular updation of quality standards and use of improved assay technology are important aspects for effective management of blood bank activities. An instrument like ECL 760 will be a great support for the study of quality parameters as well as for the establishment of good transfusion practices.
References:
- Nicholas Hooper; Tyler J. Armstrong; NCBI Books: Hemorrhagic Shock: May 2019(https://www.ncbi.nlm.nih.gov/books/NBK470382/ )
- Gunjan Bala, Anshul Gupta, Vijay Suri, Sahil Chhabra, Shaffy, Ramit Gupta; Quality Control of Fresh Frozen Plasma using Factor VIII and Fibrinogen Levels as Measure: One Year Study in a Tertiary Care Hospital; International Journal of Contemporary Medical Research; Volume 6 | Issue 8 | August 2019
- Standard for blood banks and blood transfusion services; National AIDS Control Organisation (NACO) 2007
- User information guide for Erba Thrombin reagent
- User information guide for Erba Factor VIII deficient plasma
- Mitu Dogra, Meena Sidhu, Rahul Vasudev, Ashu Dogra; Comparative analysis of activity of coagulation Factors V and VIII and level of fibrinogen in fresh frozen plasma and frozen plasma; Asian Journal of Transfusion Science - Vol 9, Issue 1, January - June 2015