Diagnosing Hepatitis B Infection
The scourge of Hepatitis
Humans are the only reservoir of Hepatitis B Virus (HBV), which is 50 to 100 times more infectious than HIV. Despite vaccinations, Hepatitis B Virus (HBV) infection continues to be a global health issue. The World Health Organization (WHO) reports that approximately 2 billion people worldwide have been infected with HBV and approximately 350 million individuals suffer from HBV-induced chronic liver disease. The prevalence of HBV infection varies in different parts of the world, with the largest disease burden being in Asia and Africa.
HBV infection
HBV infection can occur as an acute or chronic disease. The acute presentation can be life-threatening due to massive liver damage from the host immune system. Chronic HBV infection is the most common presentation of the disease. These chronic HBV infections can be associated with high levels of viral replication, with few or no symptoms. Without intervention, 15% to 40% of chronic HBV-infected individuals develop cirrhosis, end-stage liver disease, Hepatocellular Carcinoma (HCC), or require liver transplantation.
In endemic areas, most HBV infections occur at birth or during early childhood. HBV is spread through contact with infected serum, sexual activity, unsafe transfusions and vertical transmission from mother to infant. An HBV infection passed from mother to child, poses higher probability of the child becoming a chronic, asymptomatic carrier. An accurate diagnosis of infected patients is therefore, essential to the prevention of transmission and effective treatment.
Laboratory Diagnosis of HBV infection – Serological markers
There are 2 HBV specific antigens that can be detected directly in the serum of an infected person, namely – HBsAg (Hepatitis B surface antigen) and HBeAg (Hepatitis B envelope antigen).
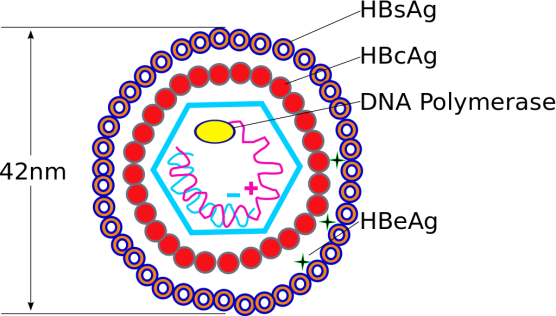 The HBV particle
The HBV particle
HBsAg is the major viral immunogenic protein, which is found on the surface of the viral particle and can be detected in the serum as a diagnostic marker of active HBV replication. Its presence in the serum for more than 6 months indicates a chronic HBV infection. The presence of HBeAg also indicates high level of viral replication. Some variants of HBV do not produce HBeAg, however the patient continues to have high levels of HBsAg.
Several specific antibodies can also be detected in both active and chronic HBV infections. The presence of anti-HBs (antibody against HBsAg) indicates either a past HBV infection or recent vaccination. Anti-HBe (antibody against HBeAg) is detected only during acute HBV infections.
Table 1: Diagnostic Assays for HBV
| Assay Type |
Name |
Use |
| HBV antigen |
HBs Antigen |
Detected in active HBV infection |
| |
HBe Antigen |
Detected during high level of HBV replication |
| HBV antibody |
Anti-HBs |
Detected in immune individuals due to immunization and/or infection |
| |
Anti-HBc IgM |
Detected in a new HB infection |
| |
Anti-HBc total |
Detected in individuals with a current HBV infection or past infection |
| |
Anti-HBe total |
Detected after recovery from an acute infection |
| HBV molecular |
Quantitative HBV DNA |
Detected in an early HBV infection and used to monitor HBV-infected patients during antiviral treatment |
HBV - Genetic variability:
HBV is classified into genotypes and sub-genotypes. Accordingly, eight major HBV genotypes (A-H), two novel genotypes (I & J), and several sub-genotypes are well described. HBV strains are also characterized into nine major HBsAg subtypes based on their antigenic determinant. The HBsAg, which is the most immunogenic protein of HBV, has a single major antigenic determinant, called the ‘a’ determinant. Mutant HBV strains pose a challenge to the diagnosis of HBV infection. Mutations inducing a conformational change within the ‘a’ determinant may result in a variant epitope of the HBsAg that is not recognized by some diagnostic assays.
A number of studies have evaluated the available HBsAg diagnostic assays, to determine the ability to detect well-defined HBsAg mutants.
Mutations within this region change the ability of the protein to be detected by monoclonal antibodies. Thus, the use of monoclonal antibody to capture HBsAg may not be sufficient to detect altered HBsAg associated with mutations. A more robust approach is to use a pool of well-characterized polyclonal antibodies directed to HBsAg. Polyclonal antibody based assays appear to detect mutant HBsAg as well.
Transasia Bio-Medicals Ltd. has developed advanced HBsAg ELISA kits, ErbaLisa PICO HBsAg and ErbaLisa SEN HBsAg, with a pool of Polyclonal antibodies coated on the microwells to maximize the sensitivity for HBsAg mutants. The conjugate reagent contains monoclonal antibodies specific to HBsAg, thereby ensuring better specificity too.
ErbaLisa SEN HBsAg and ErbaLisa PICO HBsAg also offer the best in class analytical sensitivity for 0.1 ng/ml and <50 pg/ml respectively with a turnaround time (TAT) of only 75 minutes, thereby making them convenient and reliable for use in screening for HBV infection.